Abstract
Haploidentical hematopoietic cell transplantation (HaploHCT) using high-dose post-transplant cyclophosphamide (PTCy) has been increasingly used in patients with hematologic disorders with promising results. However, limited data are available on the incidence, pattern, and risk factors including donor/recipient KIR genotypes for cytomegalovirus (CMV) reactivation after HaploHCT with PTCy. Furthermore, the impact of CMV reactivation on HaploHCT outcomes is not yet well-described. In this retrospective study, we evaluated a series of 119 consecutive patients who underwent HaploHCT with PTCy at City of Hope for hematological diseases, between January 2009 and December 2016. CMV reactivation was monitored by our institutional PCR assay (quantitative detection limit: 500 gc/ml, qualitative limit: 250 gc/ml) at least once a week for 100 days post-transplant, with preemptive anti-CMV therapy for positive PCR according to our institutional guidelines.
The median age of the cohort was 43 years (range: 2 to 71 years); with 47 female and 72 male patients. CMV serostatus of donor/recipient was Donor−/Recipient− (D−/R−) in 7, D+/R− in 6, D−/R+ in 23, and D+R+ in 82 patients. Patients received fully ablative (n=46) or reduced intensity/non-myeloablative conditioning (n=73) followed by peripheral blood stem cell (n=81) or bone marrow (n=38) graft from sibling (n=42) or non-sibling haploidentical donors (n=77). Graft-versus-host disease (GVHD) prophylaxis was PTCy plus tacrolimus/mycophenolate mofetil. Diagnoses of these patients were acute leukemia (n=80), bone marrow failure (n=15), lymphoma (n=11), chronic leukemia (n=6), hemoglobinopathies (n=5), and multiple myeloma (n=2), and the HCT-comorbidity index was more than 2 in 42% (n=50) of patients.
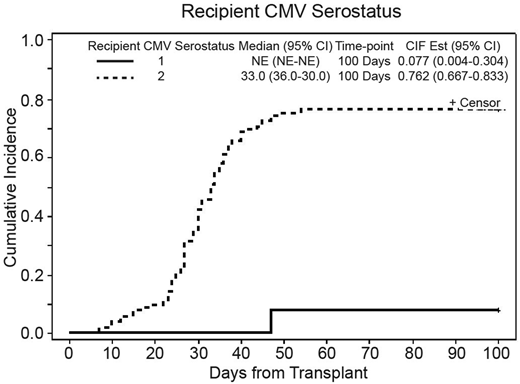
Cumulative incidence (CI) of CMV reactivation for the entire cohort was 68.1% (95%CI: 58.8-75.7%) at 100-days, with the median time to reactivation at 35 days (95%CI: 33-40); 76.2% in seropositive recipients and 7.7% in seronegative recipients (p<0.001). (Figure 1) Among 81 patients with any CMV reactivation; median initial viral load was 624 copies/mL (range: <500- 9930) and peak viral load was 2601 copies/mL (range: <500-166,865) with median CMV area under the curve (AUC) of 28,002 (range: 1671.0-2,149,929.5). In seropositive recipients (n=105), CI of CMV reactivation was 76.5% (95%CI: 66.9-83.6%) with the median onset of CMV reactivation at 33 days (95%CI: 30-36). Majority of patients (n=62) experienced only one reactivation episode with the median duration of 17 days (range=1-62) while 16 patients experienced more than one reactivation episodes with the median duration of 63.5 days (range: 33-78). Prolonged CMV exposure (>17days of CMV viremia) was seen in 47 patients.
In univariate analysis; recipient CMV serostatus, recipient sex, and stem cell source were statistical significant factors, affecting CI of CMV reactivation, but only recipient CMV serostatus stood as an independent factor on multivariable analysis (HR=15.5, 95%CI: 2.3-106.3 for R+ compared to R−, p=0.005) (Figure 1). We also evaluated the effect of KIR status on the CI of CMV reactivation. Multivariable analysis indicated a trend towards decreased incidence of CMV reactivation in donors with 2DS5 and 3DS1 (HR= 0.71 for both donors; p value= 0.1). Donor-recipient KIR mismatch was also associated with a trend towards increased incidence of CMV reactivation (p=0.1) and statistically significant prolonged CMV reactivation (OR=2.48, 95%CI: 1.12-5.47, p=.025).
With median follow up duration of 24.3 months (range: 10.3 to 99.2) and in univariate analysis, CMV reactivation, higher peak CMV PCR, time to CMV reactivation, or prolonged exposure to CMV were not associated with worse overall survival (OS), relapse-free survival (RFS), relapse incidence, or non-relapse mortality (NRM). Interestingly, the risk of extensive chronic GVHD was greater in patients who had no CMV reactivation than those who developed prolonged/recurrent CMV reactivations (41.9% vs. 26.2%, p= 0.046)
In conclusion, recipient and not donor serostatus is predictive of CMV reactivation in HaploHCT with PTCy. With the current pre-emptive therapy approach, CMV reactivation did not translate into higher rate of CMV disease or worse survival outcome. Donor-recipient KIR match (KIR licensing) and donor activating KIR genes (2DS5 and 3DS1) may be involved with CMV control after HaploHCT.
Dadwal:MERK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Shire: Research Funding; AiCuris: Research Funding; Gilead: Research Funding. Ali:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees. Salhotra:Kadmon Corporation, LLC: Consultancy. Forman:Mustang Therapeutics: Other: Licensing Agreement, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal